Embark on an enlightening journey with our elements compounds mixtures worksheet answer key, an invaluable resource meticulously crafted to illuminate the fundamental principles of chemistry. This comprehensive guide unravels the intricate world of elements, compounds, and mixtures, empowering you with a profound understanding of their properties, interactions, and applications.
Our meticulously curated content unveils the essence of each topic, providing lucid explanations and thought-provoking insights. Dive into the captivating realm of chemistry as we explore the building blocks of matter and unravel the mysteries that govern their behavior.
Understanding Elements, Compounds, and Mixtures
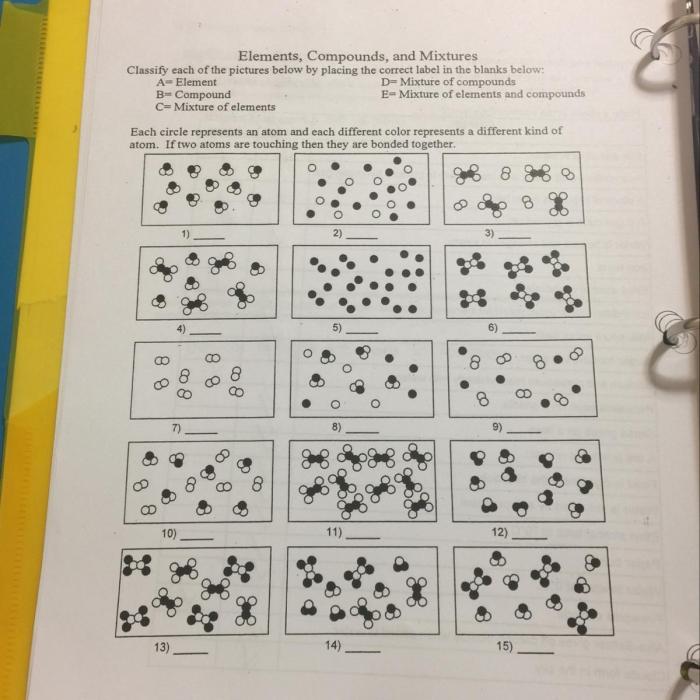
In chemistry, matter can be classified into three fundamental categories: elements, compounds, and mixtures. Understanding the differences between these categories is crucial for comprehending the structure and properties of substances.
Elementsare the simplest form of matter and cannot be broken down into simpler substances by chemical means. They are represented by symbols consisting of one or two letters, such as H for hydrogen or Fe for iron.
Compounds, on the other hand, are formed when two or more elements chemically combine in fixed proportions. They have unique properties that differ from their constituent elements and can be represented by chemical formulas. For example, water (H2O) is a compound composed of hydrogen and oxygen.
Mixturesare physical combinations of two or more elements or compounds that are not chemically bonded. They can be separated into their components by physical means, such as filtration or distillation. Unlike compounds, mixtures do not have a fixed composition and their properties are an average of the properties of their components.
Identifying Elements, Compounds, and Mixtures, Elements compounds mixtures worksheet answer key
Identifying elements, compounds, and mixtures is essential for understanding their properties and behavior. Here are some key characteristics:
- Elements: Cannot be broken down further by chemical means; represented by chemical symbols.
- Compounds: Formed by the chemical combination of elements; have unique properties and chemical formulas.
- Mixtures: Physical combinations of elements or compounds; can be separated by physical means; have variable compositions.
Q&A: Elements Compounds Mixtures Worksheet Answer Key
What is the difference between an element and a compound?
An element is a pure substance that cannot be broken down into simpler substances by chemical means, while a compound is a substance composed of two or more elements chemically combined in fixed proportions.
How can I identify a mixture?
Mixtures are typically heterogeneous, meaning they have varying compositions and properties throughout. They can often be separated into their component parts by physical means, such as filtration or distillation.
What are the different types of chemical reactions involving elements, compounds, and mixtures?
There are numerous types of chemical reactions, including synthesis, decomposition, single-replacement, double-replacement, and combustion reactions. Each type involves specific changes in the composition and structure of the reactants and products.